
Bringing more COVID-19 testing to Africa
Posted on Thursday, January 27, 2022
By
Topic: Environment and social responsibility
With so few Africans vaccinated against COVID-19, simple and sensitive tests are critical for saving lives. NEB is donating reagents to aid in the development of diagnostic tests (cLAMP) that can be used instead of PCR and can deliver rapid results detected by a simple color change. They are ideally suited for resource-poor areas by not requiring expensive laboratory equipment.
The state of pandemic efforts in Africa
COVID-19 testing is a major challenge in Africa. In October of 2021, the World Health Organization (WHO) released an assessment of testing in Africa, in which it estimated that 6 out of 7 COVID-19 infections go undetected. By October 10th of 2021, Africa had administered about 70 million tests to its population of 1.3 billion. For a point of reference, the U.S. had administered over 550 million tests to its population of 331 million, and the United Kingdom had administered 280 million tests to its population of 56 million.
Vaccination rates also lag with most African nations failing to reach the 40% vaccination target set by the WHO by the end of 2021. Only about 9% of Africans were fully vaccinated by the end of 2021.
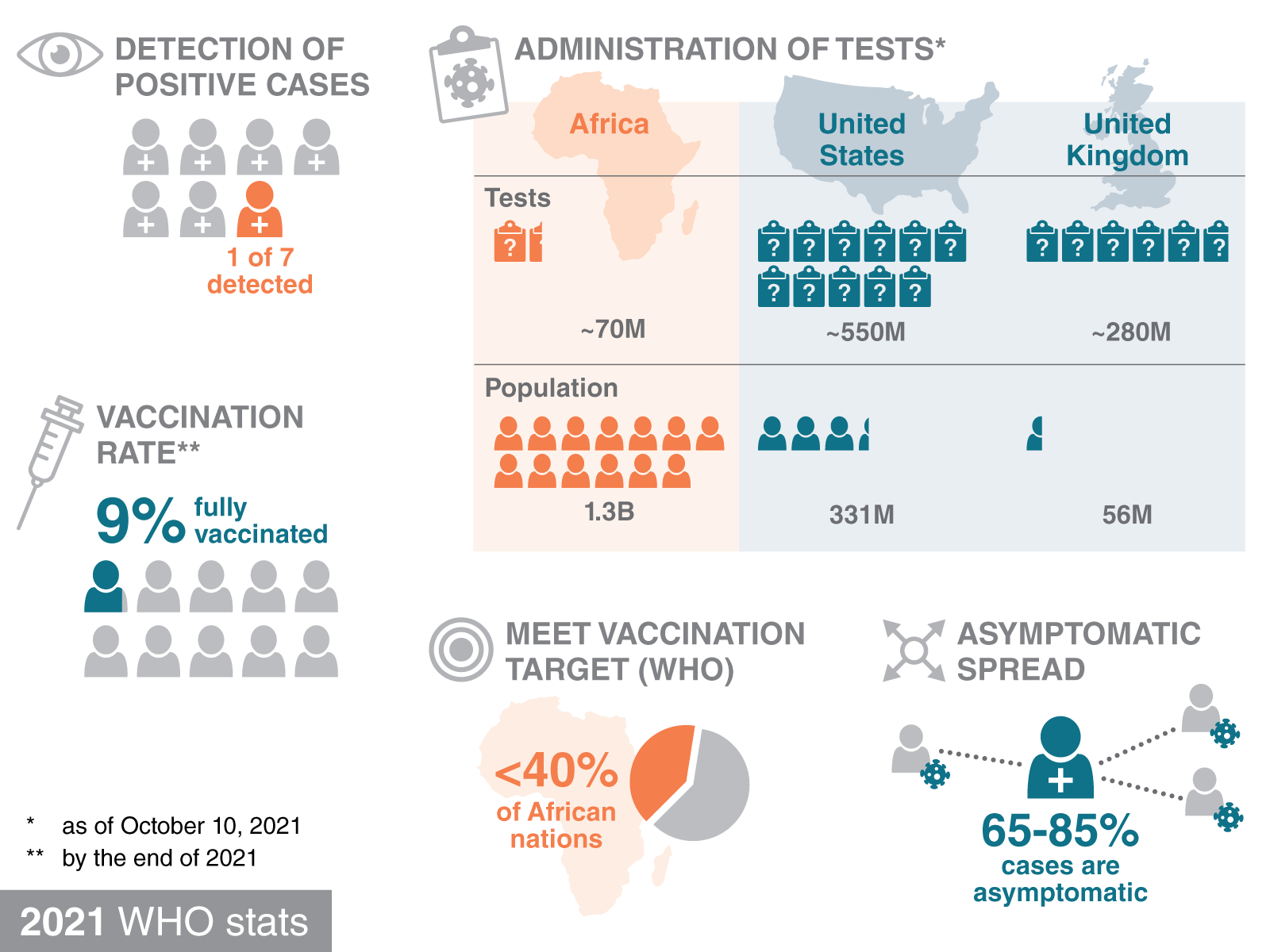
Without higher vaccination rates, increased testing can be used to protect communities from high rates of viral spread. Estimates indicate that 65% – 85% of the cases are asymptomatic, making it easy for infected individuals to spread the virus without their knowledge. To put it bluntly, the lack of inexpensive COVID-19 testing is a big problem in Africa.
African labs are successfully piloting RT-LAMP for molecular diagnostics
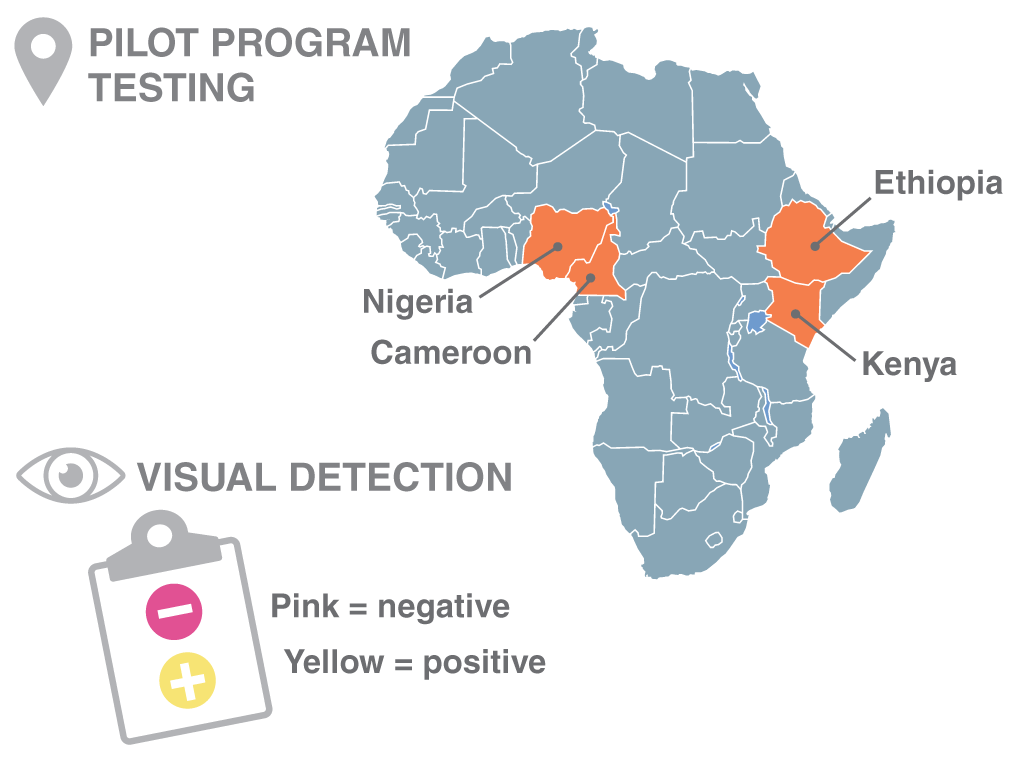
New England Biolabs® (NEB®) has collaborated with the International Centre for Genetic Engineering and Biotechnology (ICGEB) to help create an alternative method to diagnose COVID-19 based on the use of the reverse transcriptase loop-mediated isothermal amplification (RT-LAMP) assay. RT-LAMP is a rapid isothermal amplification technology requiring minimal equipment to carry out, making it an ideal method for locations with limited testing infrastructure. A multicenter observational pilot study has been completed in the African countries of Cameroon, Ethiopia, Kenya, and Nigeria, which demonstrated high specificity and sensitivity of a colorimetric RT-LAMP assay for COVID-19 detection. An expanded Phase II program will soon begin, with reagents donated by NEB, and funding by the Bill & Melinda Gates Foundation.
Test results with a simple change in color
Loop Mediated Isothermal Amplification (LAMP) Tutorial

Overall, continued efforts are needed to enhance testing accessibility within African countries. Africa represents an important part of the global community and access to testing and vaccines impacts the state of the COVID-19 pandemic around the world. The success of alternative low-cost, rapid diagnostic methods in Africa could be a blueprint for other nations struggling to meet their population’s COVID-19 testing needs.
NEB will not rent, sell or otherwise transfer your data to a third party for monetary consideration. See our Privacy Policy for details. View our Community Guidelines.
Products and content are covered by one or more patents, trademarks and/or copyrights owned or controlled by New England Biolabs, Inc (NEB). The use of trademark symbols does not necessarily indicate that the name is trademarked in the country where it is being read; it indicates where the content was originally developed. See www.neb.com/trademarks. The use of these products may require you to obtain additional third-party intellectual property rights for certain applications. For more information, please email busdev@neb.com.
Don’t miss out on our latest NEBinspired blog releases!
- Sign up to receive our e-newsletter
- Download your favorite feed reader and subscribe to our RSS feed
Be a part of NEBinspired! Submit your idea to have it featured in our blog.


