Large scale mRNA synthesis could be streamlined with thermostable RNA polymerase research advance
Posted on Tuesday, September 8, 2020
By
Topic: What is Trending in Science
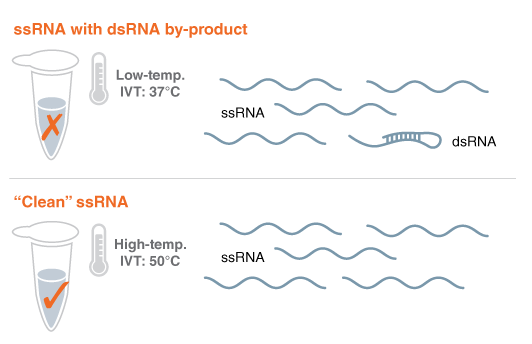
Figure 1: Using thermostable RNA Polymerase to eliminate problematic RNA byproducts and avoid lengthy purification
What are some of the biomedical therapeutics that would use in vitro transcription (IVT) produced synthetic RNA?
In the past five years or so, one of the most prominent uses of synthetic RNA, has been to generate messenger RNA, or mRNA. The idea here is very simple. You make RNA in a tube then introduce it into a cell to hijack the cell's machinery to make any protein you are interested in. The cell itself acts as the bioreactor to make any protein of interest. All you need is the DNA sequence to make the RNA that you are interested in. The rest of the process is very generic. You use the same process for any protein. The only thing that you're changing is the input DNA sequence.
Some of the most prominent biomedical applications for IVT are for therapeutics and vaccines. It's currently being evaluated for use in vaccines for infectious diseases, cancer immunotherapy, and replacing defective proteins. Let me give you an example – once the sequence of the COVID-19 viral genome was deciphered, DNA sequences of interest (that could be a potential vaccine target) were synthetically generated and mRNA was synthesized in tubes; these mRNAs are currently being evaluated as a vaccine.
Why does synthetic RNA produced from RNA polymerase trigger an immune response?
IVT is a very robust, efficient process to synthesize RNA, yet under certain circumstances, the RNA polymerase can have spurious activities. It makes the RNA that you are interested in, but at the same time, it also makes some unwanted byproducts. The RNA product isn’t always clean. For certain applications, it really doesn't matter but for in vivo applications it is critical. One of the biggest criteria for any biologic is that it must be very precise and clean. Let's say 99% of your RNA is the RNA of choice, 1% percent is a byproduct. That byproduct could be problematic for in vivo applications.
The goal for synthetic mRNAs that are made with IVT is to look like a natural RNA, an endogenous mRNA. The problem is that the RNA polymerases that are currently used, T7 RNA polymerase (#M0251), has spurious activity. It makes the single-stranded RNA of choice, but some of the RNAs are a little longer. Those long RNAs can fold back and form double-stranded RNA. Normally the cell doesn't have this double-stranded RNA. The cell knows that this is unusual. It's a foreign entity that it is encountering, and that activates an immune response. For certain cases, you would want to have that immune response but for certain kinds of therapeutics, that immune response is detrimental.
Would it be correct to say that this double-stranded RNA might be viewed by the cell as a virus?
Yes, it can be mistaken as viral dsRNA. This is the same immune response that's triggered when you have a viral infection. Some viral genomes can be double-stranded RNA. The cell sees the byproduct and triggers an immune response against the synthetic RNA, thinking that it is encountering a viral genome. It's natural for the cell to fight against anything that it doesn't think belongs there, right?
Why is immunogenicity considered a problem in mRNA vaccines? Aren't all vaccines supposed to drive an immune response?
Immunogenicity from the mRNA, depending on the therapeutic application, can either be beneficial or detrimental. As you said, it can be potentially advantageous for vaccines because it can act as an adjuvant to elicit immune responses. However, immune sensing of the mRNA (due to the by-products) has been observed to be inhibitory for antigen expression and it will negatively impact the actual immune response that you want to trigger from the vaccine per se. This phenomenon is not understood completely but it is an important question in the field.
How does your group's high-temperature IVT process using thermostable T7 RNA Polymerase simplify reducing immunogenicity?
The double-stranded RNA byproduct is a big problem in the field. When I started my group here at NEB a couple of years back, I decided to tackle that double-stranded RNA issue first. It was interesting from a scientific perspective to understand how it's made, but also key for the application of this technology. At that time using synthetic RNA for therapeutics was gaining traction.
We decided to take complementary approaches. First, we wanted to understand the double-stranded RNA byproducts, how they are made, their source, and what they look like. Second, we wanted to figure out how we can reduce formation of the double-stranded RNA formation.
We figured out that there are two different kinds of byproducts made in the reaction. The predominant one is the one that causes immunogenicity for the most part. Once we knew their nature, it was easier, scientifically to come up with a solution to prevent their formation.
In the research division at NEB, we had a research program to engineer thermostable RNA polymerases, (i.e. Hi-T7 RNA Polymerase #M0658), that allow you to perform the IVT reaction at higher temperatures than normal. We observed that this prevented the formation of those double-stranded RNA byproducts and reduced the immune response. It was nice to be able to speculate and achieve that result, based on our complementary research approach, and then see it happen.
How does this new process support scale-up for RNA vaccines?
That's a great question because scaling up is one of the biggest issues for the researchers using synthetic RNA to develop COVID-19 vaccines. The first step is to find a vaccine that will work. The second step is figuring out how you are going to make milligrams, grams, kilograms of that RNA.
Avoiding an expensive purification process is how our method could help to scale up manufacturing of mRNAs vaccines. Conventionally, purification is implemented downstream of the IVT reaction to get rid of the problematic RNA byproducts from the reaction. It's not very cost-effective. Our goal is to come up with a novel platform that streamlines the process.
What we did was to compare the immune response of high-temperature-produced RNA with RNA purified by the conventional process. We saw the reduction in immune response without having to perform the extensive downstream purification step. By preventing RNA byproduct formation in the first place, you can avoid the need for that time-consuming post synthesis purification process.
That's terrific. What a timely finding! Could this new molecular technique have an application for RNA vaccine development?
Yes, it's very timely. It's always been an exciting field, but today, many groups are pushing the limits of this technology for developing a vaccine for COVID-19.
The answer is yes, it could be used, but it can be improved. Our new process is the first step, not the final version. Now that we understand the mechanism, we want to show a head-to-head comparison with the standard process. Another goal is to take it as close as possible to the current RNA synthesis workflow. We are striving to take those next steps in this exciting project that could hopefully support therapeutic RNA synthesis efforts.
What is the key idea that scientists should know about your research?
People have been using RNA polymerases for decades, RNA synthesis works great. But at the same time, now that the applications are evolving for use in human therapeutics, we need to understand more about the enzymes that are involved in this process to meet that higher set of criteria.
My group is working to hit that sweet spot between understanding the mechanisms and improving the applications. This use of thermostable RNA polymerase is the tip of the iceberg. Therapeutic and vaccine applications of synthetic RNA open many mechanistic questions about RNA polymerases. Immunogenicity from dsRNA is just one aspect. There are other criteria that need to be addressed as well. In the future, we may not be making RNA the same way that we make it today. We hope to be able to innovate new methods and introduce new workflows using new or improved enzymes, just as we have done with this study.
The deeper we look at the RNA polymerases, the more we will understand, and discover to help make the RNA synthesis process more efficient for vaccines and therapeutics.
NEB will not rent, sell or otherwise transfer your data to a third party for monetary consideration. See our Privacy Policy for details. View our Community Guidelines.
Don’t miss out on our latest NEBinspired blog releases!
- Sign up to receive our e-newsletter
- Download your favorite feed reader and subscribe to our RSS feed
Be a part of NEBinspired! Submit your idea to have it featured in our blog.




