Periplasmic protein folding
A significant portion of the enzymes produced in E. coli at NEB are involved in nucleotide modification which can be toxic when expressed in the cytoplasm, resulting in poor purification yields. One simple solution is to export these proteins to the periplasm. However, this compartment has not been extensively developed for overexpression of recombinant proteins. Since the periplasm lacks ATP and is permeable to the environment, ATP-independent chaperones and folding factors are necessary. By linking heat shock protein folding stress to a genetic selection, we are able to capture and characterize novel protein folding solutions.
Using this strategy, we have uncovered new periplasmic folding solutions:
a) Transcriptional factors which reprogram the proteome of E. coli to facilitate the periplasmic folding of recombinant proteins
b) Cytoplasmic chaperones which assist folding through unknown mechanisms when expressed in the periplasm
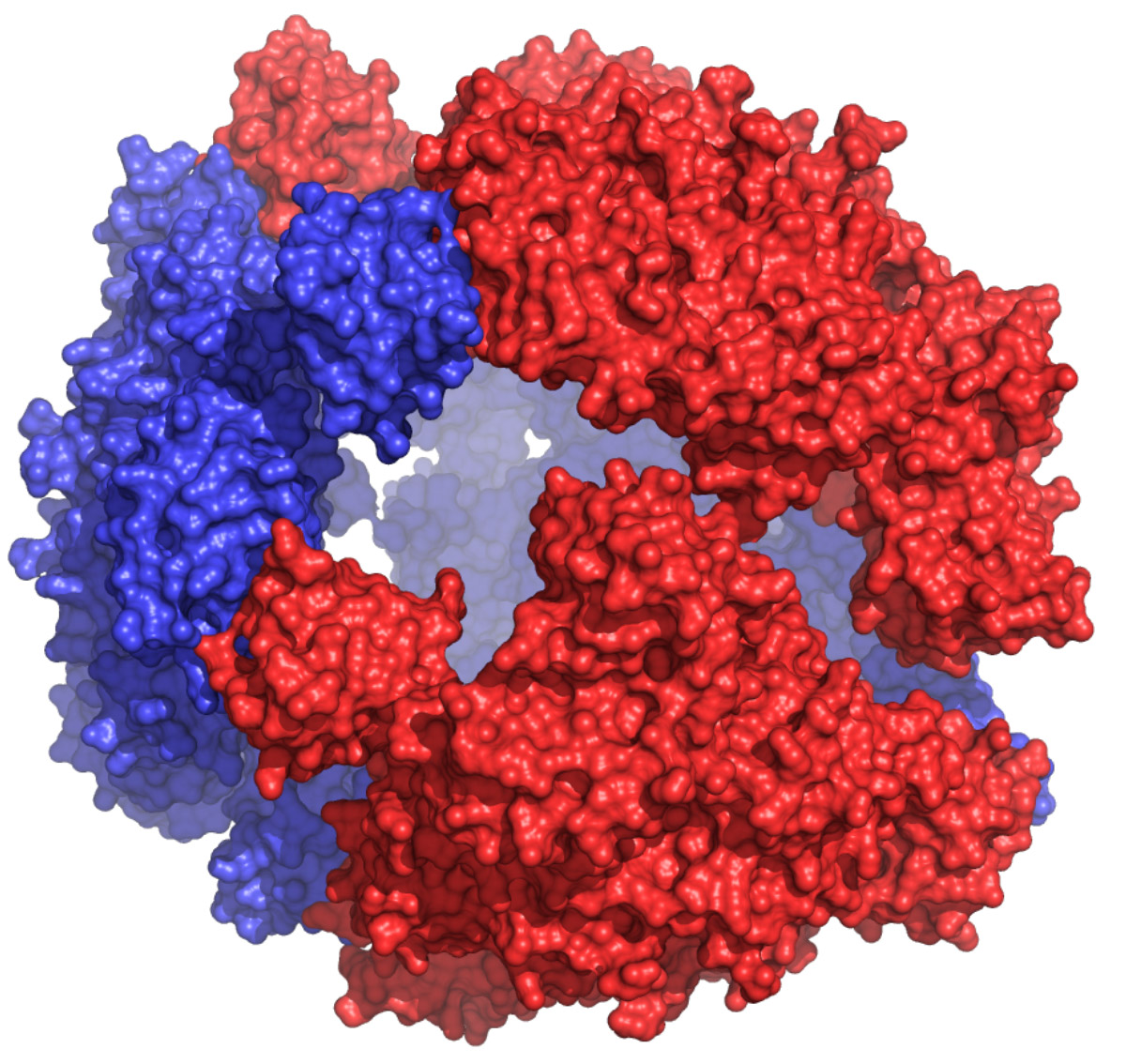
Cage-like structure of DegP
DegP is a periplasmic protease/chaperone involved in envelope protein homeostasis. In times of envelope stress, DegP trimers assemble around unfolded proteins to form a large 24-mer cage (DegP24), enclosing an inner cavity ~8 times larger than that of GroEL, with the potential to accommodate up to 300 kD proteins (PDB: 3OU0).

